Male Hormone Optimization for Brain Health, Sleep, and Longevity
Hormone Replacement Therapy, or Hormone Optimization Therapy, as Dr. Amy Killen suggested that we call it at the recent Longevity Global Summit at the Buck, changed my life for the better. It radically improved my sleep, increased my energy, made it easier to exercise, and lifted my mood.
About 1/2 of NeuroAge’s clients have trouble sleeping. Poor sleep is truly an epidemic for people’s longevity. In the ITP studies (large well done mouse studies), estradiol replacement is one of the only and largest effects on mouse lifespan (second to rapamycin), and it only has an effect in male mice. I have found this intriguing for some time.
All of these threads got me thinking about whether hormone replacement is as helpful for sleep, brain health, and longevity for men as it is for women.
Here I synthesize current evidence on hormone optimization for brain health and longevity, covering testosterone, estrogen, DHEA, and DHT/5α-reductase inhibitors.
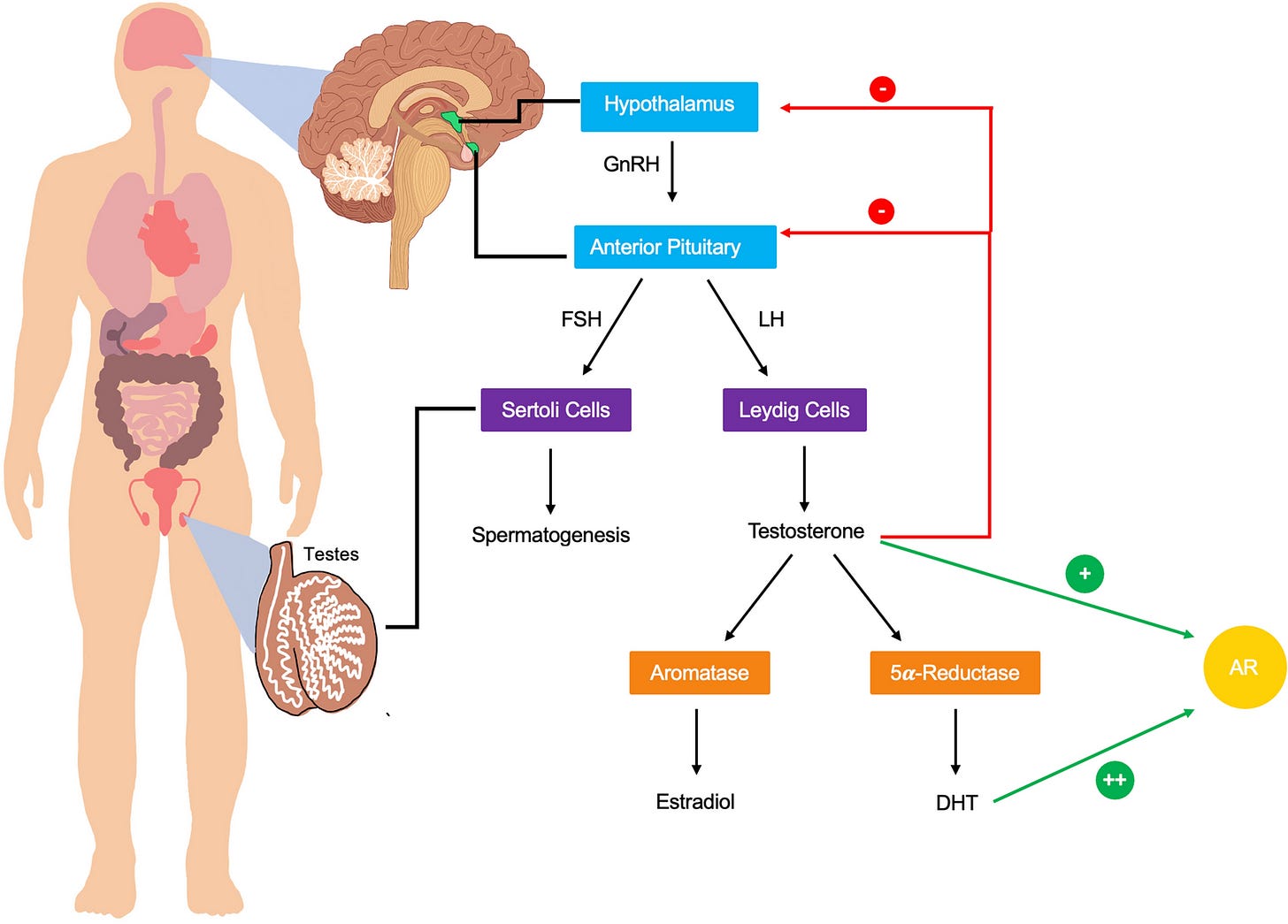
First some anatomy and pathways of male hormone synthesis and regulation for your reference:
Age-Related Changes in Hormones
Understanding which hormones actually decline with age—and which do not—is essential for evidence-based optimization.
Key insight: Total estradiol stays stable because increased aromatase activity in aging fat tissue compensates for declining testosterone. Progesterone does NOT decline with age in men (contradicts some functional medicine claims).
Part I: Testosterone Replacement Therapy
Mechanistic Pathways for Neuroprotection
Testosterone acts on the brain through well-characterized molecular pathways. Androgen receptors are densely expressed in the hippocampus, cerebral cortex, and amygdala. Primary mechanisms include: amyloid-beta clearance (30-40% reduction via neprilysin upregulation), neurogenesis enhancement, mitochondrial function improvement (PGC-1α, PINK1/Parkin pathway), and anti-inflammatory effects.
Epidemiological Evidence
Multiple large prospective cohort studies associate low testosterone with 14-80% increased dementia risk. The UK Biobank (159,411 men) found HR 1.43 for all-cause dementia and HR 1.80 for Alzheimer’s in the lowest testosterone quintile. (PubMed)
The Evidence Gap: RCTs Show No Cognitive Benefit
The NIH Testosterone Trials enrolled 493 hypogonadal men with memory impairment. After 12 months, there was no significant difference in any cognitive measure. A 2019 meta-analysis of 23 RCTs (1,438 participants) found negligible cognitive effects (Hedges g = 0.09). (PMC)
*A Note on RCTs vs. Observational Studies in General
The differences in results from Randomized Control Trials (RCTs) and observational studies has caused controversy in many different domains including optimal cholesterol levels and statin use. RCTs are widely recognized as the gold standard and a higher level of evidence than observational studies because they are carefully controlled with placebo groups, blinded, and constructed to avoid confounding factors. If RCTs show signal, that is generally considered strong evidence.
However, if they are negative, there can be many reasons why this is the case. Low subject numbers and short studies chief amongst them. Just look at the above RCT meta-analysis numbers for testosterone replacement and cognition- 23 RCTs only had a total of 1,438 men- that’s 100X fewer participants than the UK Biobank observational study (159,411 men), which means that it is much less powered to see statistical differences. Also the RCTs are comprised of 23 small studies- on average each one had only 62 subjects. Combining small studies together can increase noise because they are all designed a bit differently- different cognitive measurements (some of which might show signal and some not), different study populations (some of which might show signal and some not). Lastly, some effects take time and if a study was only 3 to 12 months long, it may not be enough time to see benefits unlike the decades long observational studies.
This is why I don’t discount large observational studies when the RCTs don’t pan out with small numbers. Small RCTs are a data point but not the final word and observational studies are a data point but also not the final word. For testosterone optimization and brain health, I think the jury is still out and we need larger longer RCTs to have a clearer picture.
APOE4 Interaction
The Vietnam Era Twin Study (717 men) found the cognitive difference between low and high testosterone corresponded to ~8 years of cognitive aging in ε4 carriers vs only 2.3 years in non-carriers. (PMC)
TRT Shows Complex, Dose-dependent Effects on Sleep
Testosterone and sleep share an intimate physiological relationship. In healthy men, 60-70% of daily testosterone production occurs during sleep, with levels peaking during the first REM sleep episode approximately 90 minutes after sleep onset. This rise requires at least three hours of uninterrupted sleep with normal architecture—sleep fragmentation significantly attenuates nocturnal testosterone secretion (Wittert, Asian Journal of Andrology, 2014).
Clinical trial evidence presents a nuanced picture. The landmark Testosterone Trials (TTrials) involving 788 men over age 65 found that 12 months of testosterone gel did not significantly improve fatigue scores on the FACIT-Fatigue scale, though modest improvements occurred in mood and vitality (Snyder et al., New England Journal of Medicine, 2016). However, the EARTH study in Japan demonstrated that TRT significantly improved sleep disturbance over 12 months in hypogonadal men without pre-existing sleep apnea (Shigehara et al., Aging Male, 2018). The key distinction appears to be patient selection and baseline sleep status.
Sleep Apnea Risk Demands Careful Monitoring
The most significant concern with TRT involves obstructive sleep apnea (OSA). High-dose testosterone administration (500mg weekly) in older men shortened total sleep time and worsened sleep apnea by increasing the oxygen desaturation index by 10% (Liu et al., Journal of Clinical Endocrinology & Metabolism, 2003).
The Endocrine Society guidelines strongly recommend against initiating TRT in patients with untreated severe OSA, while European Academy of Andrology guidelines take a more permissive stance, recommending monitoring rather than absolute contraindication. Clinically, screening for OSA symptoms before TRT initiation, avoiding therapy in severe untreated cases, and monitoring for worsening symptoms represents the current standard of care.
TRAVERSE Trial: Cardiovascular Safety
The TRAVERSE trial (5,246 men, 33-month follow-up) found TRT was better than placebo for cardiovascular events (HR 0.96). However, new safety signals emerged: atrial fibrillation (+46%), Venous Thrombotic Embolism (VTE, +46%), and paradoxically increased fractures (HR 1.43). (PubMed). This suggests that men on TRT should be monitored for these potential safety signals. Also estrogen levels should be monitored and not pharmacologically manipulated to levels that are too low (see below).
Part II: Estrogen in Aging Men
Estrogen is often overlooked in male hormone optimization. Men produce estradiol primarily through aromatization of testosterone. Normal levels: 10-40 pg/mL.
Bone Health: Estrogen Matters More Than Testosterone
Approximately 70% of bone resorption is estrogen-dependent in men. The MrOS Sweden Study found fracture risk increases exponentially when estradiol falls below 16 pg/mL. (PubMed)
Cardiovascular U-Shaped Mortality Curve
The JAMA Jankowska study (501 men with heart failure) established a striking U-shaped relationship: men in the lowest estradiol quintile (<12.9 pg/mL) had 317% higher mortality than those in the optimal range (21.8-30.1 pg/mL). Men in the highest quintile had 133% higher mortality. (JAMA)
Cognitive Effects: Testosterone’s Benefits May Depend on Estrogen
The Cherrier study randomized 60 men to testosterone, testosterone + anastrozole (anastrozole blocks the aromatization of testosterone to estrogen), or placebo. When aromatization was blocked, verbal memory improvements disappeared. (Neurology). This tells us that the cognitive benefits from TRT could be due to estrogen replacement, not testosterone replacement itself.
Estrogen's Role in Male Sleep: Underexplored but Clinically Relevant
Estradiol, produced primarily through aromatization of testosterone, has significant neuromodulatory effects in sleep-regulatory brain regions. Men actually have more estrogen receptors in the brain’s sleep centers than women.
Despite this receptor expression, observational studies consistently show that men’s sleep appears less sensitive to estradiol fluctuations than women’s. Analysis of NHANES 2013-2016 data involving 5,406 adults found no significant association between sleep duration and serum estradiol in American men across all age groups (Zhu et al., International Journal of Endocrinology, 2025). Similar null findings emerged from a study of 531 Singaporean men (Goh & Tong, Journal of Andrology, 2010).
Animal research provides mechanistic insight despite limited human data. Castrated male rats given estradiol showed enhanced wakefulness during active phases, higher EEG theta power, and improved REM sleep recovery after deprivation—suggesting potential benefits in androgen-deprived states (Wibowo et al., Behavioural Brain Research, 2012). However, comparative studies confirm that the magnitude of sleep changes induced by gonadal steroids is consistently greater in females than males.
For men on TRT, estradiol management requires balance. Aromatase inhibitors used when E2 exceeds 40-60 pg/mL can cause insomnia when they suppress estradiol too aggressively. Clinical practice suggests optimal E2 levels of 20-40 pg/mL with a testosterone-to-estradiol ratio of 10-30, though no randomized trials have established sleep-specific estradiol targets.
Mouse Lifespan Studies: 17-α-Estradiol
The NIA Interventions Testing Program found 17-α-estradiol extended male mouse median lifespan by 19%—one of the largest effects documented—with no effect in females. Castration eliminated all male-specific effects, indicating interaction with testicular hormones. (PMC)
Part III: DHEA (Dehydroepiandrosterone)
DHEA is the most abundant steroid hormone in humans, secreted by the adrenal zona reticularis (adrenal= above kidneys, a small area at the top of your kidneys). Levels peak at age 20-30 and decline by up to 80% by age 75. Both DHEA and DHEA-S are neurosteroids, which are synthesized in the brain.
Mechanistic Rationale for Neuroprotection
Anti-glucocorticoid: Attenuates cortisol-induced suppression of neurogenesis
BDNF modulation: Increases brain-derived neurotrophic factor expression in rats (PubMed)
Receptor modulation: NMDA receptor agonism (facilitates memory), GABA-A receptor antagonism (increases neuronal firing)
Neuroprotection: Against oxidative stress, excitotoxicity, and oxygen-glucose deprivation
RCT Evidence: Small Studies are Negative
DAWN Trial (2008): 225 healthy adults aged 55-85 randomized to 50mg DHEA vs placebo for 1 year. No differences in any of 6 cognitive function tests. (PMC).
No major medical organization recommends DHEA for cognitive enhancement. However, it is notable that no further RCTs have been run since 2008 so data is very limited.
The DHEA-S:Cortisol Ratio as a Biomarker
While DHEA supplementation has not shown benefit so far, the DHEA-S:cortisol ratio may be more useful. Optimal ratio ~5:1 to 6:1. High ratio associations: chronic stress, cognitive deterioration, immune dysfunction, accelerated epigenetic aging. A 2025 study found cortisol/DHEA-S ratio was the best predictor of epigenetic age acceleration (Pubmed).
Part IV: DHT and 5α-Reductase Inhibitors
Dihydrotestosterone (DHT) is the most potent androgen, formed from testosterone by 5α-reductase. 5α-reductase inhibitors (finasteride, dutasteride) are widely prescribed for hair loss and BPH (benign prostatic hypertrophy, the age-related enlarging of the prostate that makes many older men have to urinate frequently)—but disrupt neurosteroid synthesis including allopregnanolone.
5α-Reductase Inhibitor Safety Concerns
Sleep Concerns with 5α-Reductase Inhibitors
While DHT itself lacks strong direct evidence for sleep regulation, the 5α-reductase enzyme is the rate-limiting step for synthesizing several sleep-promoting neurosteroids, particularly allopregnanolone.
Allopregnanolone is a potent positive allosteric modulator of GABA-A receptors with “somnogenic properties very similar to short-acting benzodiazepines” (Neuropsychopharmacology, 2001). By blocking 5α-reductase, finasteride and dutasteride deplete this neurosteroid in both plasma and cerebrospinal fluid (Caruso et al., 2015; Melcangi et al., 2017).
Pharmacovigilance data reveal concerning signals. Analysis of FDA Adverse Event Reporting System data from 2004-2017 found:
Insomnia: Reporting odds ratio 1.93 (95% CI 1.77-2.09, P<0.0001)
Obstructive sleep apnea: Reporting odds ratio 5.65 (95% CI 4.83-6.62, P<0.0001)
Survey data from post-finasteride syndrome patients show over 50% report chronic insomnia that persists after drug discontinuation (Ganzer et al., 2015).
Notably, one polysomnography study found no differences in sleep spindle morphology between finasteride users and controls (Plante et al., Human Psychopharmacology, 2016), and the REDUCE trial of 6,914 men found dutasteride improved nighttime urination frequency but had no effect on sleep quality over four years (Kuhlmann et al., Journal of Urology, 2021). This discrepancy between pharmacovigilance signals and controlled trials likely reflects both selection bias in voluntary reporting and the possibility that effects occur in susceptible subpopulations.
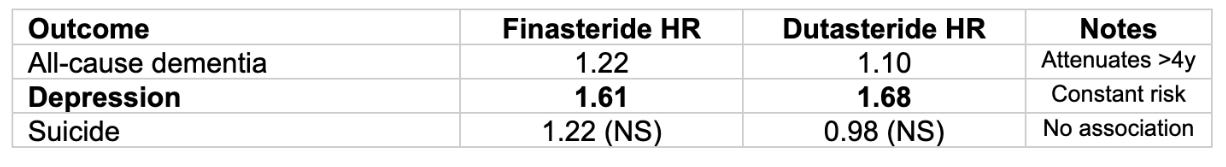
Depression and Dementia Concerns with 5α-Reductase Inhibitors
A large Swedish cohort study (2.2 million men) examined neurological outcomes, demonstrating increased risk of dementia (notably this went away after 4 years of use) and consistent increased risk of depression.
Source: Garcia-Argibay et al., JAMA Network Open 2022. Depression risk remained constant over time. Dementia associations attenuated after 4+ years, suggesting possible detection bias.
Part V: Clinical Decision Framework
Biomarker Targets
Aromatase Inhibitor Management
Core principle: Don’t use prophylactically. Only ~2.6% of men on TRT need AIs.
When to consider: E2 >60 pg/mL (with or without symptoms), or 40-60 WITH symptoms
Try first: Lower T dose, increase injection frequency, switch to topical, weight loss
Critical floor: NEVER suppress below 20 pg/mL—accelerates bone loss, increases CV/cognitive risks
Conclusions
Testosterone: TRT is reasonable for symptomatic lower testosterone men seeking healthspan optimization. RCT evidence does not support use specifically for cognitive protection but larger observational studies and mechanistic animal studies do. The jury is still out on cognitive benefits and dementia prevention with TRT. TRT appears positive for sleep but sleep apnea should be carefully monitored.
Estrogen: Adequate estrogen (20-30 pg/mL) is equally important as testosterone for bone, cardiovascular, and cognitive health. Aggressive suppression with aromatase inhibitors may cause more harm than benefit.
DHEA: Despite compelling mechanistic rationale, small RCTs showed no cognitive benefit. Larger studies are needed. The DHEA-S:cortisol ratio may be a useful biomarker of stress resilience and biological aging.
5α-Reductase Inhibitors: Finasteride and dutasteride carry meaningful depression risk (~60% increase) and dementia risk (reverses after 4 years of use). They may also disrupt sleep although there is a discrepancy between observational and RCT data. I hope that better drugs for BPH and hair loss emerge in the future.
The field of male hormone optimization for brain health and longevity is an emerging one and much of the data is small and preliminary. Cutting edge precision medicine for the brain will carefully balance male hormones in the future to optimize energy, metabolism, cognition, and sleep.



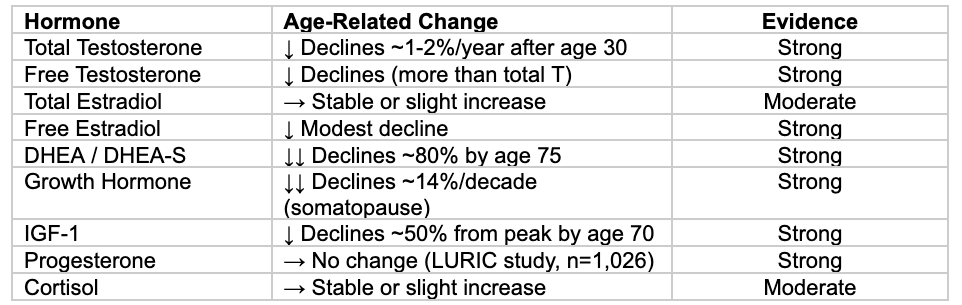


Excellent info for men. Thanks
Great comprehensive look at male hormone replacement therapy. This is the first time that I have seen adverse outcomes for 5-AR inhibitors on cognitive health. As an APOE4 carrier this is huge news, my urologist recently put me on a combo of 2 days of using Finasteride and one day using Dutasteride. I am seriously considering stopping these medications. I will begin doing a deep dive on the subject. I most likely will drop these drugs and not take the chance. I am on TRT also.