Aging biologists (biologists who study aging, who are also aging themselves) often talk, quibble really, about what constitutes a “longevity gene”. So what does this mean and why does anyone care? Longevity genes are the genetic instructions for what makes giant sequoia trees live 3,000 years, humans live 73 yrs, mice live 2 yrs, and worms live 1 month. This is important because if we can figure out what these genes are, then we can change them or change the output from them, and perhaps make humans live longer and healthier lives. How would we change them or change their output? There are a number of ways— the simplest is creating drugs that bind to and alter the protein produced by the gene in some way. Other ways are various forms of altering the gene itself or the level of output produced by it using gene therapy, RNA therapies, or CRISPR— I’ll save discussion of these topics for another post. So basically, figuring out what these longevity genes are, is the first step to life and healthspan extension.
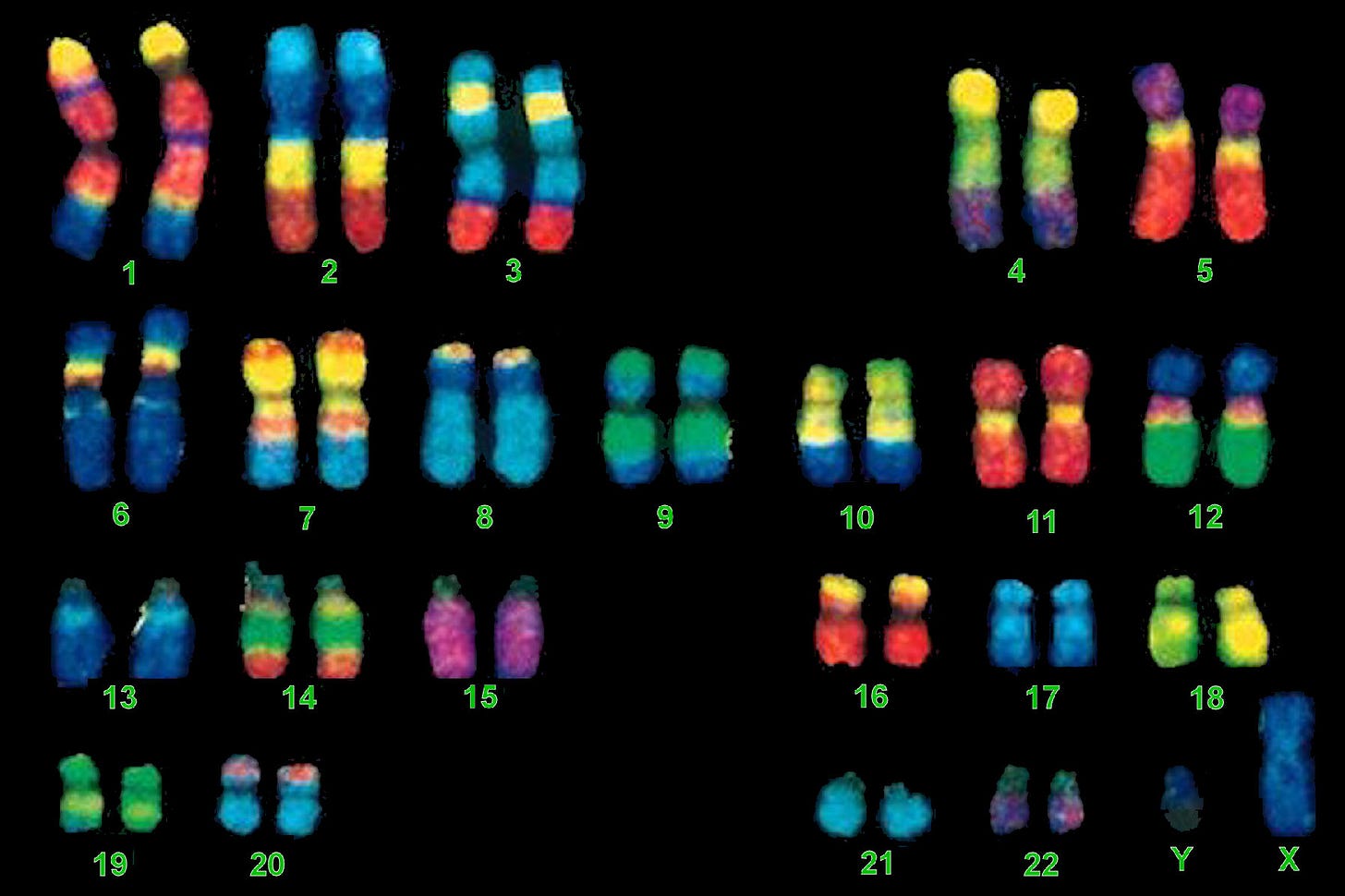
To fully understand what a longevity gene is and what we know about them so far, you first need to understand what a gene is generally. Below is an extremely magnified picture of the 23 pairs of chromosomes that reside in every cell in your body (with a few corner cases) and house your ~22,000 genes.
These ~22,000 genes each can be identified by their DNA code, which is comprised of different combinations of four “base pairs”—A,T,C, and G. Proteins in your cells read this code and manufacture RNA from it. That RNA is then turned into proteins. Those proteins then go off into your cell and perform various functions like making energy, detoxifying various things, firing in the case of neurons, opening the doors to the cell (channels) etc. Your cells are like manufacturing plants with workers (proteins) that need to be replaced often. Your genes are the blueprints for how to produce more workers. There are a bunch of ways that this is more complicated but those are the basics.
Ok so genes are blueprints for proteins that perform various functions in cells, so what? Well, most of your genes are identical to other people’s genes and are the reason that we have two hands and a nose and walk upright, etc. In fact your genes are surprisingly similar even to other species like dogs, and even worms. But what is interesting are the parts of your genes that are different from other people’s and other species’s genes. Some of those differences make one person’s eyes brown and another person’s green and one person taller and another shorter. And some of those differences in genes make some people and species live shorter or longer. That’s right, those genes, the one’s with differences in them that relate to how long an organism lives, are longevity genes.
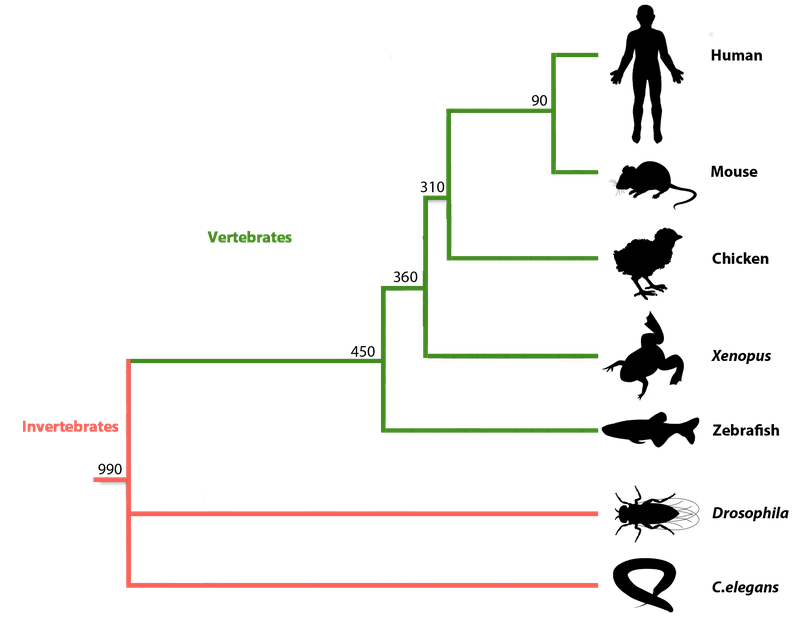
Now that we know what a longevity gene is, how do we identify it? Scientists have taken various approaches to attempting to figure out which genes are longevity genes and we do not have it entirely sorted yet. There are two main approaches to identifying longevity genes that I will discuss, bottom-up approaches and top-down approaches. Top and bottom refer to the top and bottom of the phylogenetic tree, with humans being at the top and lower organisms such as worms being at the bottom. The below figure shows a version of the phylogenetic tree with some of the organisms commonly studied in scientific labs.
I much prefer top-down approaches for reasons that I will discuss but both certainly have their advantages.
I. Bottom-up approaches
Bottom-up refers to starting with organisms that live a shorter amount of time, like yeast or worms, and performing lifespan studies to figure out the genes that cause them to live longer. Then, with the assumption (a very big assumption that I will discuss further later on) that these genes will also control the lifespan animals further up the tree and even the lifespan of humans, scientists can start moving up the phylogenetic tree testing more and more complex animals. The reason to start with lower organisms is that the lifespan experiments are shorter and cheaper than they are in mice or other higher organisms. Also you can’t very well go about mutating genes in humans without a very good reason, like imminent death from a disease, and an FDA-approved clinical trial.
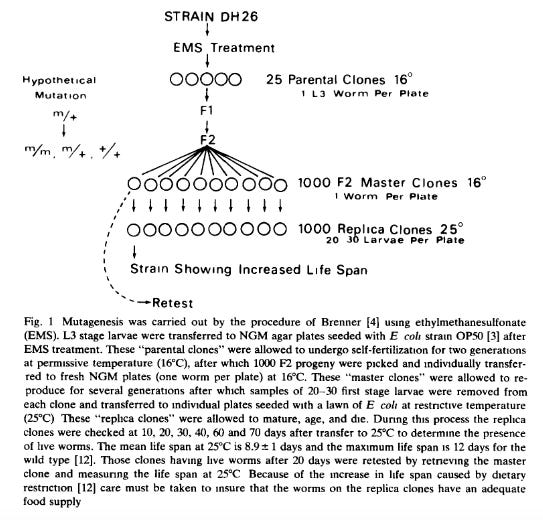
Bottom-up approaches to identifying longevity genes first started in the 1970s and 1980s. Below is a paper from Michael Klass from 1983 showing a now classical technique for “screening” the worm, C. elegans, for lifespan extension. The premise of these experiments is to mutate every gene in these worms, one by one, and see if any of the worms live longer.
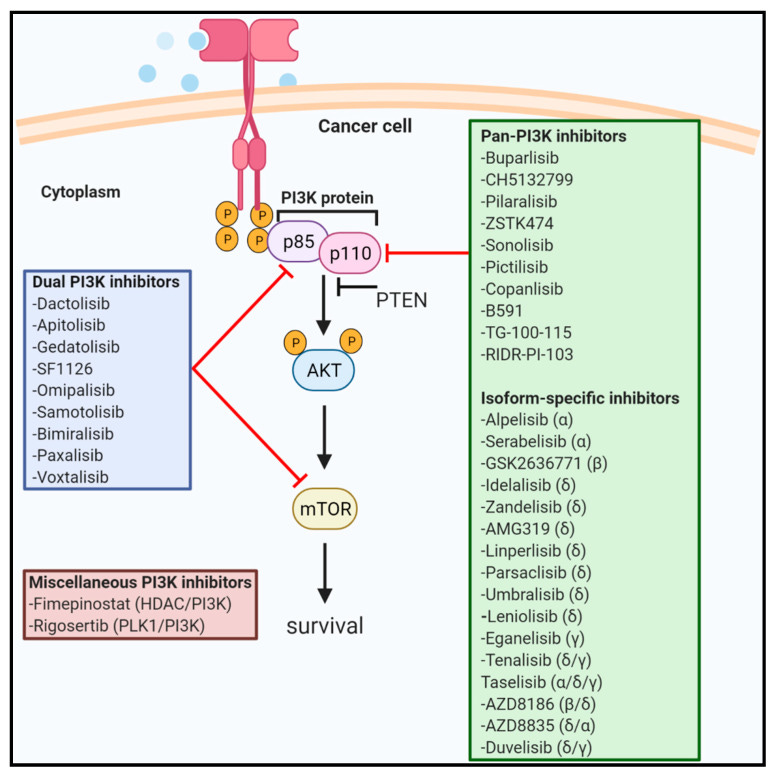
Klass identified one gene this way that some scientists believe is a longevity gene, AGE-1. Confusingly (in my opinion), the AGE-1 gene has a different name in humans, phosphatidylinositol-3-OH kinase (PI3K). PI3K is now a target of many therapeutics in the cancer space. And for those of you familiar with the drug Rapamycin, some PI3K inhibitors also inhibit mTOR (Target Of Rapamycin). So you can see how identifying the AGE-1 gene in 1983, using a bottom-up approach, has eventually led to a class of drugs to treat an age-related disease. For a nice review of PI3K inhibitors, see this 2021 paper in the scientific journal, Nature, “PI3K inhibitors are finally coming of age” (see what they did there).
Whether or not this makes PI3K a longevity gene per se, is still under debate. The gold standard, of course, would be if these drugs can extend lifespan in healthy humans (not just by being better for cancer). This leads to a whole other discussion about whether age-related diseases and aging are basically the same thing and whether the FDA should allow aging to be an indication for clinical trials. Stay tuned for that. Stay additionally tuned for posts that go into more detail on each of the genes that scientists currently believe may be longevity genes, including PI3K and mTOR.
II. Top-down approaches
Top-down approaches are essentially the reverse of bottom-up approaches. They start in humans, identify genes that may be longevity genes, and then attempt to prove this by altering those genes in lower organisms and assessing whether they live longer and/or healthier lives. How do you identify a potential longevity gene in humans? This is essentially a big data problem. Since we now can sequence the human genome pretty rapidly and cheaply— something that has only been possible in the last 10 years— we can now look at all 6 billion bases in the human genome and ask whether people with each of these bases lives longer and/or healthier lives. This approach is called a Genome-Wide Association Study (GWAS).
This has a number of advantages. You can imagine that the genes that control yeast or worm or even mouse lifespan may not be the same as the genes that control human lifespan. This is especially true when you get into organ specific healthspan- like in the case of the brain. I will probably end up talking a lot about the brain since I am a neuroscientist. C. elegans has 302 neurons and humans have 86 billion neurons. C. elegans does not get Alzheimer’s disease and neither do some more complex animals, like mice for example. So you can imagine that bottom-up approaches are going to miss a lot of important genes for lifespan and healthspan in humans that aren’t important in lower organisms. Also, many genes that are important in controlling lifespan in lower organisms may not matter that much in humans. This has been the source of much disappointment and controversy as scientists using bottom-up approaches raced up the phylogenetic tree expecting that the genes they found in lower organisms would be important for human life and healthspan.
There are also some challenges with top-down approaches- related to statistical reproducibility and multiple testing and the fact that humans, unlike inbred mice and worms, are not clones of each other and thus have a lot of statistical background noise. With some thought and in some cases, very large numbers of humans tested, these problems are rapidly being solved. I will write a future post on top-down approaches and the genetics and genomics revolution. My company, NeuroAge Therapeutics is based on technology developed during my postdoc at MIT that is on the cutting edge of these approaches.
III. Other approaches
There are some other ways besides genetics that longevity companies and academics are approaching life and healthspan extension, like eliminating senescent cells or using alternative animal models— I will cover these topics in later posts.
I would love to hear your thoughts. You can subscribe to comment or follow me @DrGlorioso or my non-profit @LongevitySF.